Product
Our ProductIntroducing RAPIDS Platform
RAPIDS is the world's first medical robotic system that can be remotely
controlled to collect specimen, therefore allowing non-contact and remote-controlled
nasopharyngeal and oral specimen collection without the risk of health workers contracting pandemic diseases.
Product Picture
Features
Medical device classification
Stereotaxic Unit, Navigation (A64110.03)
Non-contact specimen collection system,
risk-free of infection for medical personnel
Anatomical data of
the cranium allows meticulous
specimen collection
Pressure sensor and robotic
swab insertion mechanism
ensures safety
RAPIDS Platform
Preparing for post-pandemic era using medical robots
Non-face-to-face sampling prevents secondary infection, therefore strengthens medical staff’s medical capacity, and maximizes the ability of molecular diagnosis on a pandemic disease
As-is
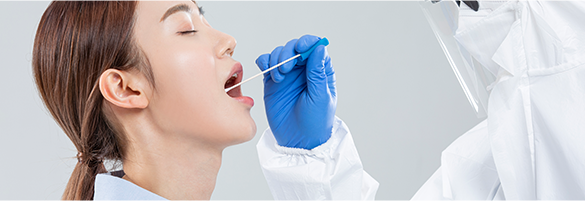
When face-to-face sampling is required by medical staff
ㆍRisk of secondary infection for medical staff increases.
ㆍIt is impossible to enter the treatment site after wearing full-body protective clothing.
ㆍIt is impossible to actively operate a clinic due to the increase/decrease in the number of people taking the pandemic disease test.
ㆍFatigue of medical staff increases due to regular operation of screening station
ㆍIt takes excessive sampling time due to large number of people who are to be tested.
To-be

Through non-face-to-face sampling
Molecular diagnosis on the pandemic disease
Preparing for post-pandemic era using medical robots
Development of a non-face-to-face automation platform
for medical staff in the entire molecular diagnosis process
for the resumption of trade with guaranteed quarantine in the post COVID-19 era

Differentiation of sampling robot function
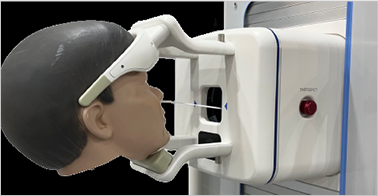
ㆍNon-face-to-face sampling, minimizing the infection risk of medical staff
ㆍSecuring field-type unmanned sampling function
ㆍMinimizing cost for mass distribution


Molecular diagnostic device for large-capacity pooling tests
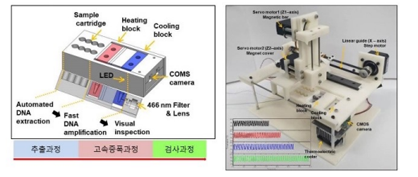
ㆍIt is possible to secure the molecular diagnostic test results of about 1,000 people within 2 hours (10test/1well)
Molecular diagnostic device for small-volume, rapid test
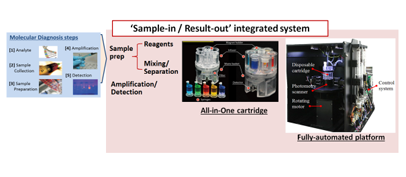
ㆍIt is possible to secure molecular diagnosis results for 12 patients within 1 hour without medical staff intervention
Plans to utilize outcomes
Needs to be Addressed

Even in military and remote locations, face-to-face sampling by medical staff is required
- ㆍDifficulty in access for sampling
- → It is required to establish mobile platforms for ships and remote locations
- ㆍLack of medical staff and medical infrastructure
- → It is required to secure a safe sampling environment through non-face-to-face control
- ㆍRisk of mass outbreak of infectious respiratory diseases
- - USS Roosevelt: 1,000 out of 5,000 crew members were infected
- - Madagascar: molecular diagnostics is limited in remote areas
- ㆍNecessity of rapid response to infectious diseases from third countries
- → Development of mobile platform that can be rapidly deployed
Solution
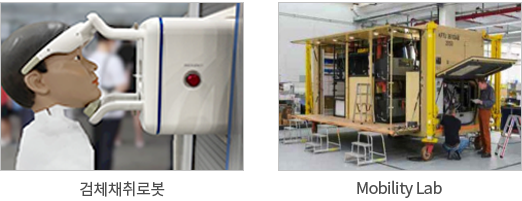
Establishing a rapid response system
through automatic molecular diagnosis
without medical staff intervention
ㆍNon-face-to-face platform to test virus in upper airway
ㆍMILSPEC response design for remote areas
ㆍPossible to deploy the lab quickly by mounting in a small container
ㆍPerforming virus research function at the site of infection
Commercialization plan
Needs to be Addressed

Departure/entry to the airport
ㆍSafe Transfeming Environment Required to Rebound Air Traffic to
Pre-COVID 19 Levels
ㆍCurrent Molecular Diagnostics Industry Cannot Support Mass Screening
Over 10,000 people
Solution
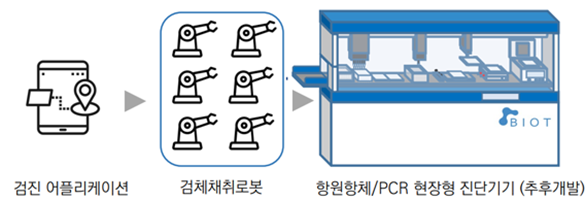
Establishment of complete molecular diagnosis
test protocol through sampling system
for inbound and outbound travelers
ㆍCheckup application
- Entering personal information of departures/arrivals, self-inquiry
- Guidance to the sampling point and to the less density area
ㆍNon-face-to-face sampling system
- Installation of multiple sampling robots suitable for the size of the inbound and outbound travelers
- Minimizing medical staff fatigue and infection risk through non-face-to-face control
ㆍAntibody/PCR field type diagnostic device (to be developed later)
- Fully automatic inspection from non-face-to-face sampling to sample handover
- Consideration of an appropriate molecular diagnostic method for a large number of people